

These patients also improved after the drug was stopped, and in 18 patients, follow-up intestinal biopsies documented recovery or improvement of the duodenum after treatment was stopped ( Mayo Clin.
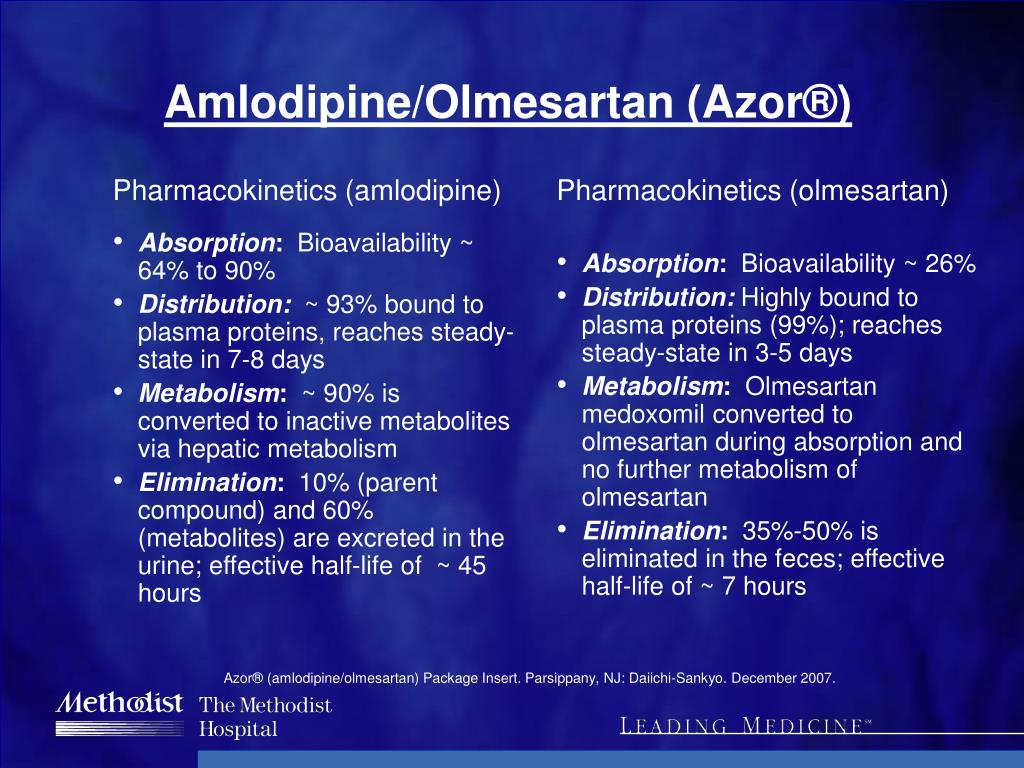
Azor side effects series#
The use of the eHealthMe site and its content is at your own risk. Every effort has been made to ensure that all information is accurate, up-to-date, and complete, but no guarantee is made to that effect.
Different individuals may respond to medication in different ways. Our phase IV clinical studies alone cannot establish cause-effect relationship. WARNING: Please DO NOT STOP MEDICATIONS without first consulting a physician since doing so could be hazardous to your health.ĭISCLAIMER: All material available on is for informational purposes only, and is not a substitute for medical advice, diagnosis, or treatment provided by a qualified healthcare provider.
Azor side effects software#
Our analysis results are available to researchers, health care professionals, patients ( testimonials), and software developers ( open API). Results of our real-world drug study have been referenced on 600+ peer-reviewed medical publications, including The Lancet, Mayo Clinic Proceedings, and Nature. We study millions of patients and 5,000 more each day. With medical big data and proven AI algorithms, eHealthMe provides a platform for everyone to run phase IV clinical trials. Hypothyroidism (abnormally low activity of the thyroid gland, resulting in retardation of growth and mental development): 1 person, 3.12%.Pyogenic Liver Abscess (liver abscess caused by bacteria): 1 person, 3.12%.High Blood Cholesterol: 2 people, 6.25%.Multiple Myeloma (cancer of the plasma cells): 18 people, 56.25%.General Physical Health Deterioration (weak health status): 18 people, 56.25%.Fatigue (feeling of tiredness): 19 people, 59.38%.Gastroesophageal Reflux Disease (a condition in which stomach contents leak backward from the stomach into the oesophagus): 20 people, 62.50%.Age of people who have Erectile dysfunction when taking Azor *:Ĭommon side effects people have besides Erectile dysfunction *:


 0 kommentar(er)
0 kommentar(er)
